This is the 21st in a series of articles that celebrate the lives of the Nobel Prize laureates whose names grace the 130+ streets of Laureate Park. These laureates are extraordinary individuals who through their lifetime achievements have made our daily lives immeasurably richer, often in ways not readily apparent. The author wishes to thank Dr. Otto Phanstiel, professor of medical education at the University of Central Florida’s College of Medicine, for his contributions to this article.
The phrase “monoclonal antibodies” has popped up in the news lately, mainly in connection with treatments for COVID-19. Those of us unlettered in science now confront an expression, like “factoid” or “gravity waves,” that we are apt to repeat without really knowing what we are saying. (Well, at least in my case.) Over the past year, several high-ranking government officials, laid low by the coronavirus, have been summarily healed after infusions of a cocktail composed of monoclonal antibodies. Closer to home, Laureate Park resident Mark Reid, cofounder of our neighborhood’s beloved Beep driverless shuttles, also found himself recently on the receiving end of that one-hour treatment delivered intravenously using a drug called bamlanivimab. But what are monoclonal antibodies, anyway? Do they have a purpose beyond treatments for the coronavirus? Let’s try to unravel a bit of the mystery behind this peculiar new addition to our everyday vocabulary while paying homage to the brilliant Argentinian biologist César Milstein, who in the 1970s invented the method for manufacturing monoclonal antibodies en masse.
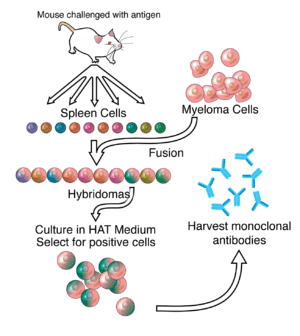
In the process devised by Milstein, the production of monoclonal antibodies starts in the spleen of a mouse. The mouse is injected with an antigen, such as a substance produced by a virus, which is recognized by the immune system of the mouse as a foreign invader.
The B cells in the mouse’s spleen react to the presence of the antigen by manufacturing antibodies to attack the intruder. The spleen is then removed from the sacrificed mouse to collect the B cells containing the antibodies. (Remember that B cells, along with T cells, are important components of the immune systems of animals and humans alike.) But B cells so extracted from the organ of a laboratory animal cannot replicate and soon die. Milstein’s ingenious innovation to overcome this obstacle was to fuse the B cells with myeloma cancer cells that do replicate easily. These fused cells, called hybridoma, are not only immortal but also multiply ad infinitum in the laboratory. In the final stage of production, the monoclonal antibodies produced by the hybridoma cells are harvested and purified for use as components in drugs to fight cancers, viruses, and other diseases.
Each antibody resembles the letter Y. The stem of the Y contains information common to all antibodies and provides structural stability to the Y shape of the antibody itself. The arms of the Y contain variable information specific to the antigen targeted and facilitate antibody binding to the antigen with high specificity. Once attached to the antigen, the antibodies enable a range of actions – depending on the antibody in question – such as destroying the targeted cell, neutralizing toxins produced by the cell, or marking the cell for destruction later by a T cell.
The decades since Milstein’s pathbreaking discovery have brought major refinements to the production of monoclonal antibodies. For example, the antibodies inhabiting the drug bamlanivimab, which was developed only months ago, are manufactured using human B cells rather than those of laboratory animals. And some pharmaceutical firms have successfully developed bispecific antibodies that can attack two separate antigens. The pharmaceutical uses for monoclonal antibodies, which already include treatments for cancer, pregnancy tests, and the detection of blood clots, continue to expand.
When you daydream about Argentina, pampas, parrillas, and the tango are likely to come to mind. And maybe soccer, too. But it might surprise you to learn that Argentina has already generated several Nobel laureates in the sciences, among whom César Milstein may be the most prominent. Born in Bahía Blanca in 1927, Milstein grew up in a humble family that nonetheless valued education highly. His Ukrainian Jewish father had immigrated at age 15 to Argentina, where he found work initially as a farm laborer and then as a salesman. Milstein’s Argentinian mother, a school principal, gave her young son a copy of Paul de Kruif’s Microbe Hunters, whose stories deeply impressed Milstein, drawing him to a career in science. In 1958, Milstein had already earned a Ph.D. in chemistry from the University of Buenos Aires when an opportunity arose to secure a British Council scholarship to pursue his studies in Britain. So off went Milstein and his biologist wife, Celia, to Cambridge University to earn a second Ph.D. There, Milstein worked with the legendary biologist Fred Sanger, one of only three individuals who have won two Nobel Prizes in their lifetimes. (For several years, Nonahood News has maintained offices in the Guidewell building on Laureate Park’s Sanger Road.)

Upon his return to Argentina in 1961, Milstein accepted a position as head of the Malbran Institute’s newly-created Department of Molecular Biology. The following year, however, a political coup ousted the democratically-elected president Arturo Frondizi, and the new regime clamped down on the nation’s scientific establishments. The director of the Malbran Institute was abruptly dismissed along with several prominent researchers. Milstein’s family name made him a potential target of Argentina’s new military leaders, who associated anyone of Jewish heritage with communism. With Fred Sanger’s invaluable help, Milstein and his wife returned to Cambridge, where Sanger gave him carte blanche to work on whatever hot topic in biology he wished. Milstein would never again reside in Argentina. During the first of four decades of his work at Cambridge, Milstein and his German colleague George Köhler devised their basic process for producing monoclonal antibodies, for which the pair shared the 1984 Nobel Prize in physiology or medicine. Milstein never formally sought a patent for his process, which peeved British Prime Minister Margaret Thatcher, who later decried the loss of those rights to American researchers.
The elegance that César Milstein employed to design his process for manufacturing monoclonal antibodies was somehow set aside when it came time for the World Health Organization (WHO) to devise a nomenclature for naming the resulting drugs. Let’s face it: Medications called ixekizumab, ustekinumab, and bamlanivimab do not gently roll off the tongue. Why, you ask, couldn’t the WHO come up with more pronounceable names? We have no clue, but we can point to a certain logic within the naming convention of, say, the drug bamlanivimab. The awkward final syllable “mab” abbreviates Monoclonal AntiBodies. The foregoing “vi” tells us that bamlanivimab fights a virus. And “bamlani” is, well, just a name. None of this will help you pronounce this tongue twister, and in fact, according to YouTube, no official pronunciation for bamlanivimab has been established, which means that you can pronounce the name of that drug any way you want. But let’s hope that you won’t have the need to say “bamlanivimab” anytime soon. But just in case you do one day require a drug derived from monoclonal antibodies and the drug works, please save a thought for that selfless South American scientist, César Milstein, who bequeathed to us the basic process for manufacturing those critically important medications.



